Urolon represents a new class of urethral injectable products: Bioresorbable Urethral Implants. It is an injectable device, indicated for submucosal implantation for the treatment of stress urinary incontinence (SUI) in adult females. It is a sterile, non-pyrogenic, totally bioresorbable, non-permanent implant, whose principle component is synthetic polycaprolactone (PCL) microspheres suspended in a carboxymethylcellulose (CMC) gel carrier (Figure 1a and 1b).
PCL is a well-known totally bioresorbable soft medical polymer. PCL has been used in numerous CE-marked and FDA approved commercial bioresorbable product applications and has demonstrated an excellent safety profile.

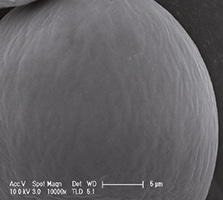
Figures 1a and 1b show light and scanning electron micrograph of PCL microspheres
